NAFDAC has issued an alert to Nigerians regarding the sale of counterfeit Tandak Injection within the country.
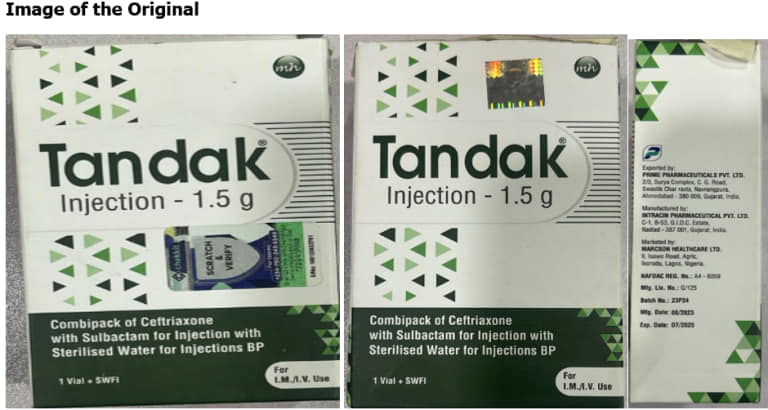
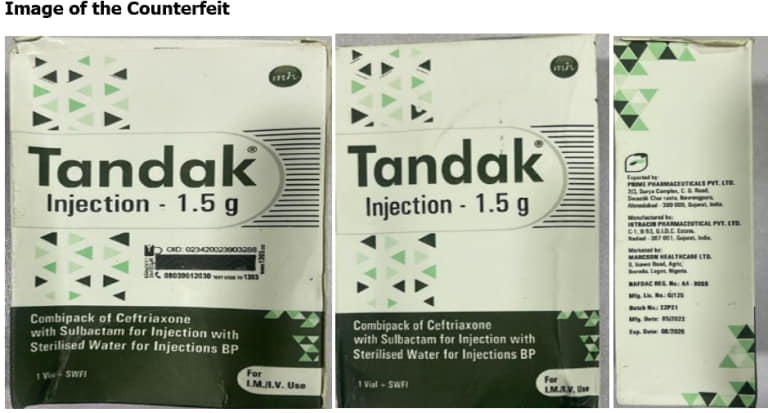
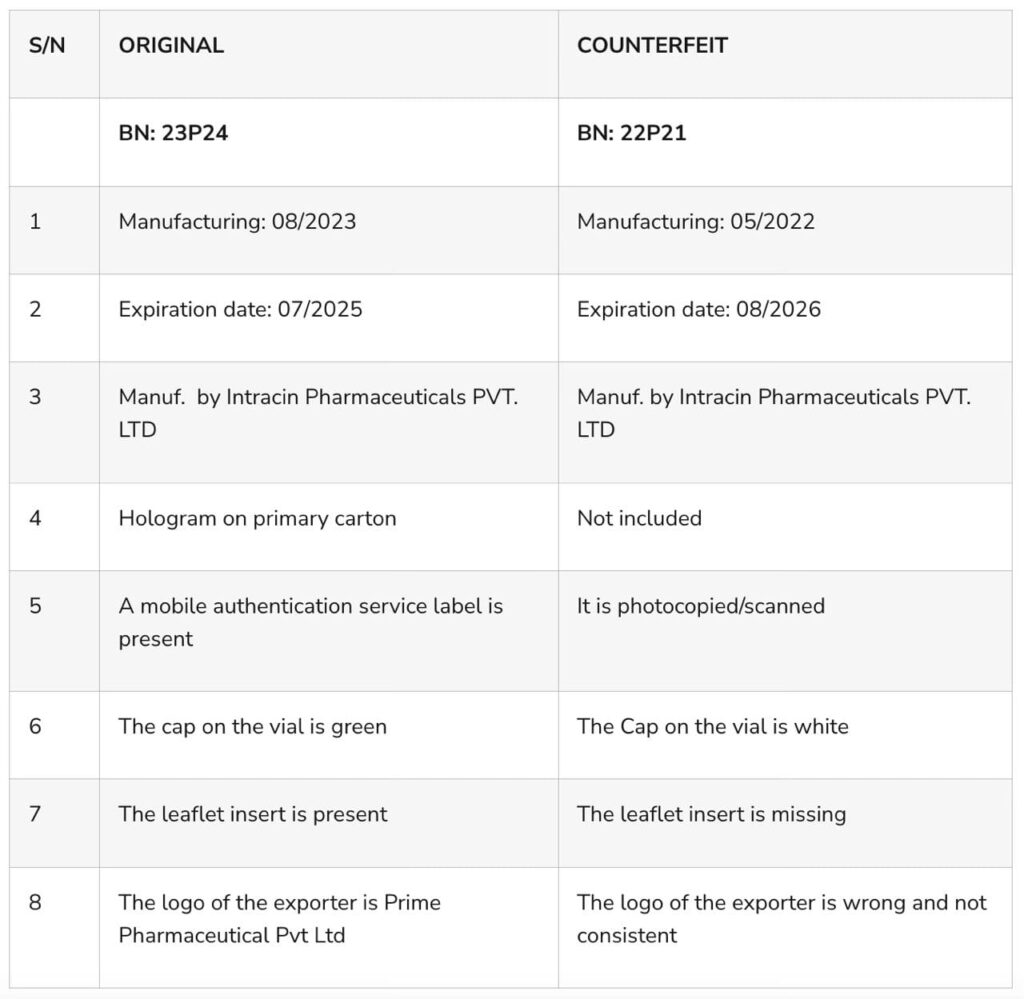
Counterfeit Tandak Injection 1.5g. The National Drug Law Enforcement Agency (NAFDAC) has issued a warning to Nigerians regarding the sale of counterfeit TANDAK injection 1.5g powder and water for injection.
The fake product was purportedly manufactured by Intracin Pharmaceuticals PVT. LTD in Gujarat, India, was uncovered in Gombe State, Nigeria.
This discovery was reported to NAFDAC by Marcson Healthcare Ltd., the Marketing Authorization Holder (MAH) for the genuine product.
‘’Tandak® injection of 1.5g powder is a co-formulation of Ceftriaxone 1000mg and Sulbactam 500mg. It is prescribed for use in the treatment of various types of bacterial infections. It fights against microorganisms by preventing their growth, and further spread of the infection. Ceftriaxone+Sulbactam 1000mg/500mg Injection should only be administered under the supervision of a healthcare professional. the statement read
The illegal marketing of counterfeit medicines poses a risk to the health of people, since by not complying with the regulatory provisions, the safety, quality, and efficacy of the products are not guaranteed.
Recommended reading: Nafdac warns against fake Tandak Injection
NAFDAC has instructed its Zonal Directors and State Coordinators to conduct surveillance and remove the counterfeit Tandak Injection from their respective zones and states.
Healthcare practitioners and consumers are urged to report any suspicion of substandard or falsified medicines or medical devices to the nearest NAFDAC office, contact 0800-162-3322, or email sf.alert@nafdac.gov.ng.





Pingback: Former Kwara Senator Rafiu Ibrahim has passed away. - Harry Deborah